BlisselⓇ was created to release women from the symptoms of vaginal atrophy
BlisselⓇ is an ultra low dose estriol vaginal gel containing 50 micrograms per dose (1 gram) of estriol. 1,2
BlisselⓇ is indicated for treating the symptoms of vaginal atrophy due to oestrogen deficiency in postmenopausal women.1
BlisselⓇ turns back the clock on vaginal changes due to the menopause:
Reversal of the cellular changes that lead to vaginal atrophy2:
- Significantly higher maturation value vs placebo (p<0.001) after 12 weeks treatment
Stops the pH changes that occur in vaginal atrophy2:
- Significantly lower pH vs placebo (p<0.001) after 12 weeks treatment


BlisselⓇ is an ultra low dose estriol vaginal gel containing 50 micrograms per dose (1 gram) of estriol. 1,2
BlisselⓇ is indicated for treating the symptoms of vaginal atrophy due to oestrogen deficiency in postmenopausal women.1
BlisselⓇ turns back the clock on vaginal changes due to the menopause:
Reversal of the cellular changes that lead to vaginal atrophy2:
- Significantly higher maturation value vs placebo (p<0.001) after 12 weeks treatment
Stops the pH changes that occur in vaginal atrophy2:
- Significantly lower pH vs placebo (p<0.001) after 12 weeks treatment
BlisselⓇ works quickly
Your patients may notice a rapid improvement in the symptoms of vaginal atrophy from the initial days of treatment.3

BlisselⓇ is effective for treating vaginal dryness
which improves or completely resolves in over 88% of women.2*
*vs placebo, P=0.001; relative risk [RR], 1.32; CI, 1.08-1.62.
BlisselⓇ significantly improves changes due to vaginal atrophy2:

Mucoadhesive Gel3
The BlisselⓇ formulation may assist the therapeutic effect whilst reducing leakage.

BlisselⓇ uses polycarbophilic components to form a mucoadhesive gel.3
This works synergistically with the estriol by providing a moisturising effect whilst increasing the retention and penetration of the hormonal component.3

Non-mucoadhesive products may have shorter vaginal contact times and produce leakage.3
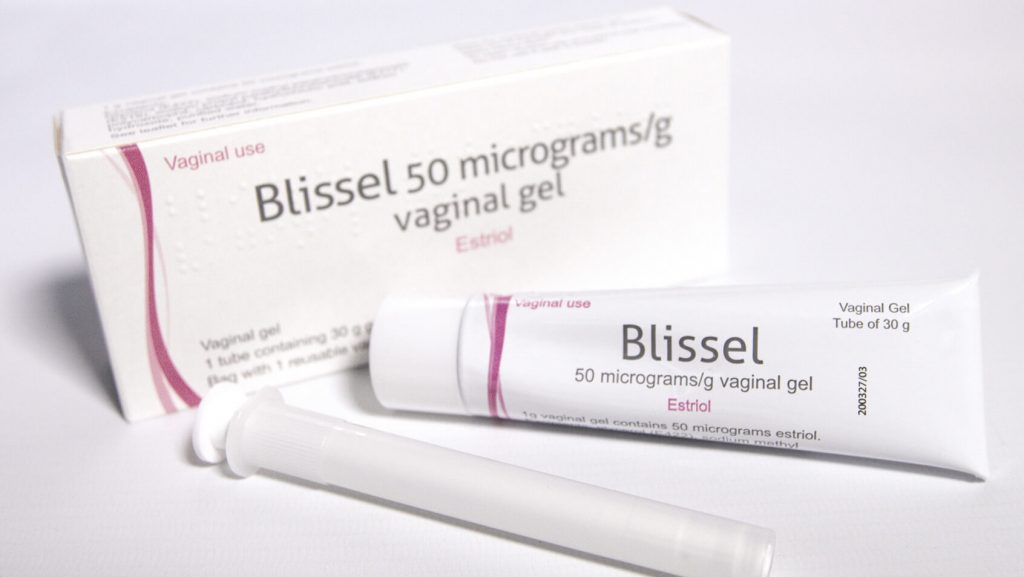
Formulation
The formulation of BlisselⓇ may allow your patients to retain more of the active ingredient, whilst reducing mess and leakage.

Ultra low dose estriol vaginal gel2
Prescribing and Adverse event reporting information for BlisselⓇ can be found here.
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Consilient Health (UK) Ltd, No. 1 Church Road, Richmond upon Thames, Surrey TW9 2QE UK or drugsafety@consilienthealth.com
- BlisselⓇ 50 micrograms/g vaginal gel. Summary of product characteristics. 2. Cano A, et al. Menopause. 2012;19(10):1130-9. 3. Lázaro-Carrasco de la Fuente J, et al. J Menopausal Med. 2022;28(2):60-69.
Date of preparation: Jan 2024 | UK-BLS-43c(1)